- Politics
- Diversity, equity and inclusion
- Financial Decision Making
- Telehealth
- Patient Experience
- Leadership
- Point of Care Tools
- Product Solutions
- Management
- Technology
- Healthcare Transformation
- Data + Technology
- Safer Hospitals
- Business
- Providers in Practice
- Mergers and Acquisitions
- AI & Data Analytics
- Cybersecurity
- Interoperability & EHRs
- Medical Devices
- Pop Health Tech
- Precision Medicine
- Virtual Care
- Health equity
A New Data Platform Aims to Build Upon ClinicalTrials.gov
Why the website’s banking on crowdsourced trial predictions, data visualizations, and community.
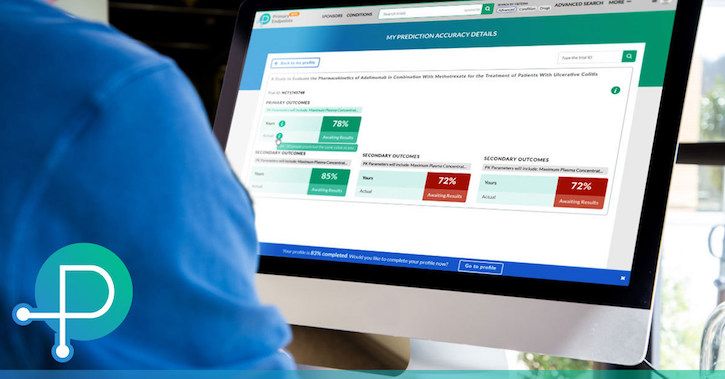
When researchers, biotech investors, and patients want clinical trial data, Scopic Software wants them to head to PrimaryEndpoints.com. The website, which remains in beta, aspires to be the “leading source” in the field, relying on a web of features and government-sourced information, according to the company.
The Massachusetts-based Scopic Software announced its clinical trial database and “collaborative platform” today, little more than a week after its launch. So what makes it stand out? Crowdsourced trial predictions, visualizations of clinical trial data, trial events and results, customized watch lists, the presence of “biotech gurus,” and user discussion boards, according to the business.
In short, Scopic Software said, the product shoots to surpass ClinicalTrials.gov, the public database fed by researchers and run by the US National Library of Medicine. Tim Burr, the site’s founder and a player in biotech, kicked off the project after “much time toiling”—and encountering problems—on ClinicalTrials.gov.
“We wanted to create a platform to ease the tedium and information overload users face when analyzing and sorting through clinical trial data,” he said. “Given its importance, clinical trial data needs to be ultra-accessible for anyone that needs it.”
Primary Endpoints uses data from ClinicalTrials.gov, which collects information by way of a mandatory reporting requirement, according to the announcement. The difference is, Burr and his team claimed, “more accessibility and ease of use.” Their desire to work around the government website echoes past complaints.
But ClinicalTrials.gov has taken steps forward in recent months. In June, for example, it trumpeted a coming series of changes geared toward improving its search, display, and information review functions. The first wave landed in beta months earlier, bringing with it what platform administrators said was a superior design and a new set of features.
Then, in late September, the website underwent additional upgrades. Taking into account feedback from stakeholders across government and industry, designers added fresh options and identifiers, like a “new” icon, to the search tool. Researchers, too, received a more accurate way to record the date when a report was published or updated, according to the government.
In the future, site designers said, they hope to enable ClinicalTrials.gov users to search for studies by city and radius, access glossary content with less struggle, and more quickly find relevant information in the study record layout.
Primary Endpoints is not the only platform to challenge ClinicalTrials.gov. State-mandated registries and other independent endeavors exist across the world. Some have sought to heighten transparency and efficiency in navigating troves of government data, while others have focused on smaller sets.
But the newcomer hopes its interface—and the community surrounding the data—will push it ahead. “People who need access to clinical trial information have been asking for a tool to simplify the monitoring and analyzation of clinical trial data,” a Scopic representative wrote in the notice, “and Primary Endpoints wants to be the answer.”
