- Politics
- Diversity, equity and inclusion
- Financial Decision Making
- Telehealth
- Patient Experience
- Leadership
- Point of Care Tools
- Product Solutions
- Management
- Technology
- Healthcare Transformation
- Data + Technology
- Safer Hospitals
- Business
- Providers in Practice
- Mergers and Acquisitions
- AI & Data Analytics
- Cybersecurity
- Interoperability & EHRs
- Medical Devices
- Pop Health Tech
- Precision Medicine
- Virtual Care
- Health equity
FDA Approves Wristband EKG Reader for Apple Watch
The Watch could tell you if your heart rate was irregular, but the KardiaBand can deliver an approved EKG in 30 seconds.
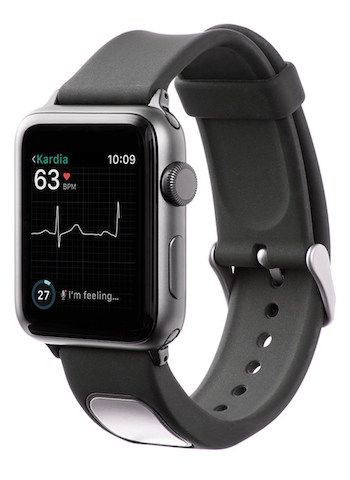
The Apple Watch may be moving closer to medical device territory, but an accessory for the gadget is already there, with FDA approval in hand.
AliveCor announced today that the FDA had cleared its KardiaBand product for marketing as a medical device. A personal electrocardiogram (EKG) monitor in the form of a basic black watchband, it can take readings in 30 seconds and display results on the Apple Watch interface through the Kardia app. Its sensor is a small silver patch along the band that collects heart rate information with a touch of the fingertip.
Apple Watch already has built-in heart rate and activity sensors that can detect possible heartbeat irregularities. AliveCor will integrate those capabilities into SmartRhythm, a new alert feature within the Kardia app. Using artificial intelligence (AI), the system will detect when heart rate and activity level seem “out of sync” and prompt the user to capture an EKG.
In a statement, Cedars Sinai Heart Institute cardiologist Dr. Ronald P. Karlsberg, MD, called the technology “a paradigm shift for cardiac care as well as an important advance in healthcare.”
Atrial fibrillation, which affects millions worldwide, is the leading cause of stroke. If the condition can be detected, 2 out of 3 strokes are preventable, but an FDA-approved EKG test is the only means of confirming the condition.
By taking EKG technology out of offices and hospitals and placing it on people’s wrists, Karlsberg said that conditions like atrial fibrillation “can be detected wherever the patient is, 24 hours a day.”
The KardiaBand builds on Apple Watch’s existing heart rate capabilities. As AliveCor CEO Vic Gundotra pointed out to TechCrunch, the smartwatch can alert users if their heart rate is high, but it can’t tell them what that really means. If the patient ends up going to a doctor or hospital as a result of that elevated heart rate, the first thing they’ll get is an EKG. The newly-approved accessory can cut the steps to diagnosis.
"These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care,” Gundotra said in the official statement.
KardiaBand is AliveCor’s second app-integrated, FDA-approved mini EKG device. Kardia Mobile, originally called the AliveCor Heart Monitor, attaches to the back of an iPhone and requires users to hold their phone with both hands for 30 seconds. Originally approved in 2012, the device also allows users to forward readings to a doctor.
The new wristband is the first Apple Watch accessory to be approved as a medical device. It will retail for $199, and require a $99 annual subscription to AliveCor's Premium service.
