- Politics
- Diversity, equity and inclusion
- Financial Decision Making
- Telehealth
- Patient Experience
- Leadership
- Point of Care Tools
- Product Solutions
- Management
- Technology
- Healthcare Transformation
- Data + Technology
- Safer Hospitals
- Business
- Providers in Practice
- Mergers and Acquisitions
- AI & Data Analytics
- Cybersecurity
- Interoperability & EHRs
- Medical Devices
- Pop Health Tech
- Precision Medicine
- Virtual Care
- Health equity
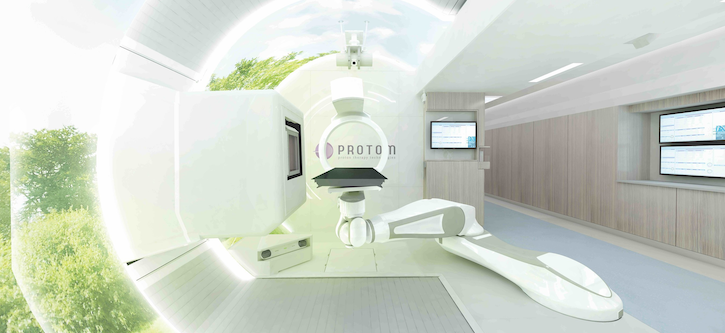
FDA Clears ProTom's Proton Therapy System
The cancer-fighting tool could give physicians more time to focus on the patient.
Photo/Thumb have been modified. Courtesy of ProTom International Holding Corporation.
The U.S. Food and Drug Administration (FDA) today announced it granted 510(k) clearance to ProTom International Holding Corporation for its proton therapy system which could improve outcomes for patients with cancer.
The Radiance 330 proton therapy system is a compact, single room system equipped with a pencil beam scanning system and integrated imaging and control system.
Pencil beam scanning is said to be the most advanced type of proton therapy and is used to treat complex cancers with extreme precision. This method uses a tumor’s locations, shape and size to create a customized pattern of protons to precisely treat the tumor and avoid nearby healthy tissue.
Using pencil beam scanning does not require patient-specific devices, which could eliminate treatment delays, reduce treatment time and costs, increase flexibility in treatment delivery and reduce exposure to secondary radiation.
Through this method, the Radiance 330 proton therapy system reduces unwanted side effects and could improve long-term patient outcomes and quality of life, ProTom said.
The system supports single, multi-room and expandable configurations and its beam transport system guides the proton beam from the synchrotron to the treatment room.
“The receipt of 510(k) clearance is the final culmination of a thorough and rigorous FDA review of the safety and effectiveness of our compact proton therapy solution,” said Steve Spotts, CEO and co-founder of ProTom International. “This achievement accelerates ProTom’s single and relentless mission to place this highly sophisticated and targeted cancer-fighting tool within reach of many more physicians.”
The system is currently installed at Massachusetts General Hospital in Boston.
Get the best insights in digital health directly to your inbox.
Related
AI Medical Service Raises $42.9M for Endoscopy Tech
FDA Clears Medical Device to Help Detect Peripheral Artery Disease
Mednition Raises $10M for AI-Powered Clinical Decision Support Tool
Podcast: Match Made in Hospitals — Patient-Matching Technology Can Improve Healthcare
September 21st 2021Clay Ritchey, CEO of Verato, highlights the administrative and financial benefits that patient-matching technology can provide hospitals and health systems, as well as how it can improve the patient experience.
Podcast: Using Digital Solutions to Address Technology Shortfalls with Citius Tech Senior VPs
July 29th 2021In an interview recorded earlier this year, Chief Healthcare Executive Associate Editorial Director Mary Caffrey spoke with 2 leaders of Citius Tech about meeting healthcare challenges with digital solutions.
